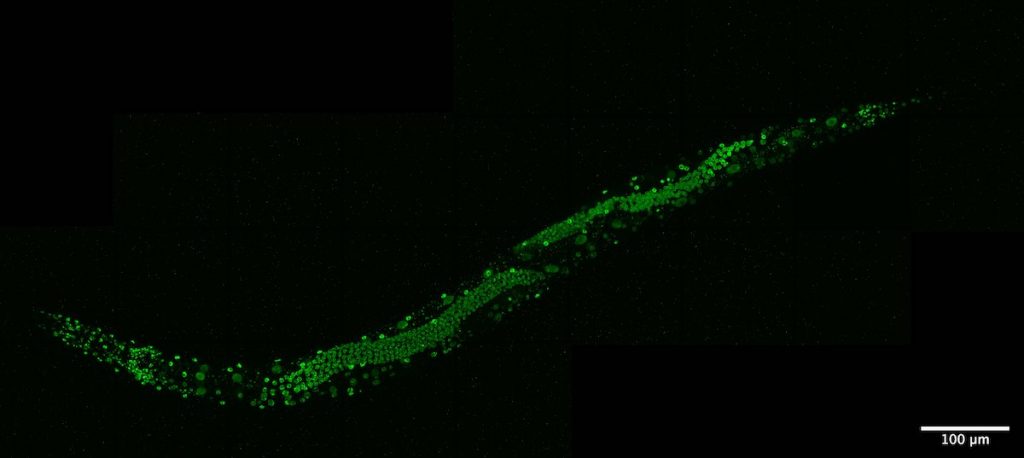
NUN bodies in C. elegans offer clues about nervous system differentiation.
One of the most active areas of research today explores the differences between individual cell types and how cells become differentiated. This specialization of cell types relies on changes in gene expression, but how those changes are orchestrated remains unknown.
Now, research published in GENETICS has identified a new organelle inside the nucleus of nerve cells in the nematode worm, C. elegans. Named “nuclear nervous system-specific bodies,” or NUN bodies, these organelles only occur in the cells of the nervous system, report Pham et al. The exact function of the NUN bodies isn’t known yet, but because these bodies are highly specific to nervous system cells, they may lead researchers to a better understanding of how these important cell types differentiate.
In their quest to unravel the mechanisms behind neuronal differentiation, the team of researchers, based at Columbia University and led by Oliver Hobert, take advantage of the simplicity of the C. elegans experimental system. The developmental fate of each of the worm’s cells is known, and the total number of distinct cell types in C. elegans is small, making it ideal for investigating tissue-specific features.
During the process of differentiation, the cells of the nervous system, known as neurons and glia, develop an unusually compact nucleus with a “speckly” appearance. This so-called granular nucleoplasm intrigued the team because there are very few morphological features that are common to all nervous system cells but absent from any other cell types.
“Why is there this nervous system specificity that’s so striking?” says first author Kenneth Pham, who was a post-baccalaureate student working in Hobert’s lab at the time. “That was the diving off point.”

Neuronal nucleus with multiple NUN bodies, as visualized with differential interference contrast microscopy. Image courtesy of the Hobert lab.
Inside the nucleus is the “control center” of the cell, where genes are turned on and off, determining the fate of the cell. Various organelles help keep things running smoothly, and it stands to reason that cells headed for different fates might have different nuclear bodies managing their gene expression patterns. To investigate, Pham set out to characterize the granules present in the nuclei of C. elegans nervous system cells.
To start with, he tested whether the granules were indeed phase-separated membraneless organelles by showing that, chemically, they behaved the same way as nucleoli. Like these known membraneless organelles, the nuclear granules dissolved in a hypertonic salt solution, and heat caused them to grow larger and decrease in number.
To make sure they weren’t just extra nucleoli, he tagged nucleolus-specific markers with fluorescent dye, revealing that each neuron contained only one nucleolus. The newly identified bodies were not nucleoli.
Pham set to work, searching for what else the granules might be. Although none are as cell type-specific as NUN bodies, a number of nuclear bodies have been described previously, such as splicing speckles, paraspeckles, PcG bodies, PML bodies, gems, stress-induced nuclear bodies, and clastosomes. He combed through the C. elegans genome, looking for any homologs of the proteins that make up these nuclear organelles in mammalian cells. By attaching a fluorescent tag to each protein, he could track whether the tag followed the NUN bodies in worm neurons. None of the six organelle components that he found in the worm genome appeared to colocalize with NUN bodies. Pham then used loss-of-function mutations in all of the organelle constituent genes that he could find, and none of them had any effect on the NUN bodies.
So, these new structures seem to be unrelated to known nuclear bodies, and what NUN bodies are made of remains a mystery. But Pham took a stab at identifying their function. In a type of cell called “canal associated neurons,” or CANs, the NUN bodies change over the course of development. At first, there may be five or six NUN bodies in the nucleus. As the worms develop, that drops to only two or three NUN bodies per nucleus, and they are enlarged.
To find out how this was happening, Pham zeroed in on genes that play a role in CAN maturation, knocking them out and looking for changes in NUN body behavior. A mutation that reduced the activity of a transcription factor, ceh-10, stopped the rearrangement of NUN bodies normally seen during development.
Ultimately, NUN bodies offer a tantalizing hint to how nervous system cells might differentiate, with lots of possible avenues for learning more. For instance, Pham began screening a mutant library to find genes needed for making NUN bodies, but so far, nothing has turned up. He’s moved on from Columbia now, beginning an MD/PhD program at the University of Pennsylvania, but he hopes someone picks up the project where he left off. “Please work on the mystery, because I really want to know.”
CITATION
A nervous system-specific subnuclear organelle in Caenorhabditis elegans
Kenneth Pham, Neda Masoudi, Eduardo Leyva-Díaz, Oliver Hobert
GENETICS, Volume 217, Issue 1, January 2021, iyaa016,