When female Anopheles mosquitoes take a blood meal from someone with malaria, a tiny Plasmodium parasite enters the mosquito’s digestive tract. That parasite can invade the mosquito’s salivary tissues, so when the insect takes another blood meal, the intruder can slip into the next human host and start a new malaria infection. Malaria is a life-threatening condition that infected 241 million people in 2020 and disproportionately affects vulnerable populations.
To combat the disease, researchers from the University of California, Irvine are developing genetically modified African malaria mosquitoes (Anopheles gambiae) that can’t transmit human malaria, alongside a gene-drive system that can quickly spread those genes and block the spread of the malaria parasite through the population. While this system usually operates with nearly 100 percent efficiency, a small number of mosquitoes will still wind up with mutant alleles that resist the gene drive. Could these mutant alleles sabotage the whole approach? In a paper published in GENETICS, Carballar-Lejarazú et al. looked at this phenomenon and found these mutations didn’t hamper the gene drive in their system.
Malaria-Resistant Mosquitoes
Contributing author Anthony James began exploring genetic methods for controlling vector-borne disease in the mid-1980s. Eventually he exploited mosquito genes that are only turned on in female mosquitoes after a blood meal and linked them with mouse antibodies that protect mice from human malaria parasites.
When these malaria-busting synthetic genes are inserted into mosquitoes, they can’t transmit malaria. And if it mates with a regular mosquito, the beneficial gene will be inherited like any other gene, gradually building up in the mosquito population. But what if that process could happen faster?
That’s when CRISPR gene editing technology hit the scene. “It seems like overnight when you work 10 or 15 years on something to make it work and then something new comes along and—in less than a year—you have it working,” says James.
Gene editing operates on the germline—the cells that will eventually become sperm or eggs—by snipping the normal chromosome and inserting the new sequence, in this case, the malaria fighting gene. In male mosquitoes, this works so well that each male passes on the new gene to nearly 100% of its offspring.
It’s a bit more complicated in female mosquitoes because egg cells are massive compared with sperm. When the system snips the normal chromosome and inserts the synthetic sequence, the second chromosome may be too far away to trigger the repair mechanism that sews the cut chromosome back up while including the system. That means there’s a chance the snipped chromosome will just stick itself back together—called nonhomologous end joining—possibly resulting in a mutant allele that resists the gene drive.
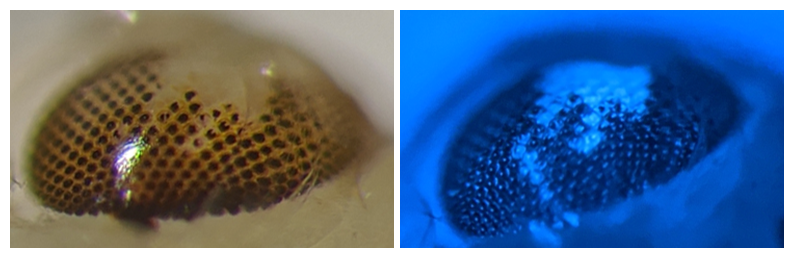
Exploring Gene Drive Mutations
To figure out if those mutant alleles could pose a problem for the gene drive system, the researchers linked the system to a somatic gene for eye color and marked it with a fluorescent protein. Then, the team performed various crosses to see how the genes passed on to future generations. Non-mutant progeny had black eyes (before adulthood) while those with the mutant allele had pink eyes. And all the progeny carrying the gene drive had eyes that fluoresced blue under light.
To make things a bit more complicated, some progeny were mosaics, with a mix of alleles and more complex eyes, but since the eye color gene isn’t part of the germline—it won’t pass on to the next generation—most of those mosaic mosquitoes still passed on the gene drive.
In the lab, about 25 percent of the first generation of progeny received the gene drive. By the fourth generation, the entire population had fluorescent blue eyes—meaning none of those mosquitoes could transmit malaria.
“Four generations is sufficient,” says James. “That’s well short of one transmission season.”
James is quick to point out that this isn’t a magic bullet for malaria and there is more research to be done. There are also many thorny issues and debates for scientists and the broader community to work through before everyone is comfortable deploying gene drive mosquitoes in the wild. But James is hopeful the project to which he’s dedicated so many years may one day help ease the malaria crisis.
“There was a famous scientist who said a new idea doesn’t take hold because you change people’s minds; it takes hold because there’s a whole new generation of people that have grown up hearing about it,” says James. “I wish it was a little faster, but we’ll do our part, and hopefully people will take it up. It may not be me, but we have something to hand off.”
CITATION:
Cas9-mediated maternal effect and derived resistance alleles in a gene-drive strain of the African malaria vector mosquito, Anopheles gambiae
Rebeca Carballar-Lejarazú, Taylor Tushar, Thai Binh Pham, Anthony A James
GENETICS
2022: iyac055
https://doi.org/10.1093/genetics/iyac055
Melissa Mayer is a freelance science writer based in Portland, Oregon.